What is the shape of the carbonate ion, (CO3)^2 ?
$ 6.00 · 5 (722) · In stock

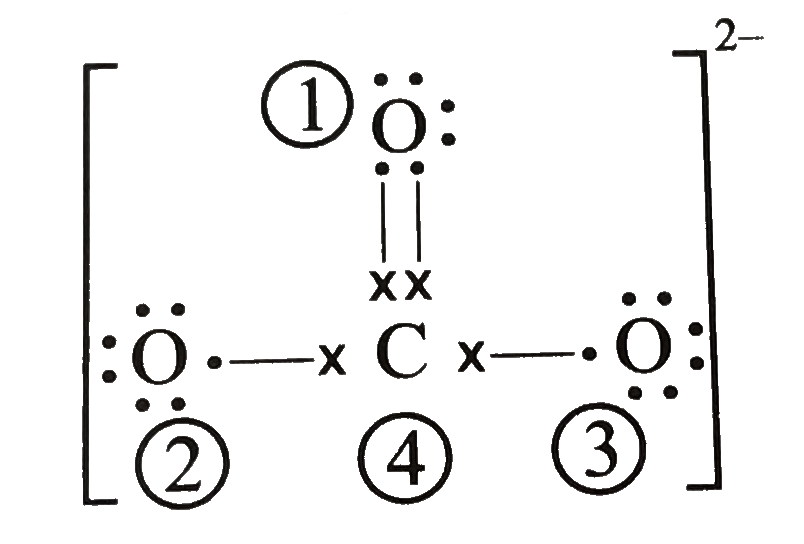
Calculate the formal charge on atoms in carbonate (CO(3)^(2-)) .
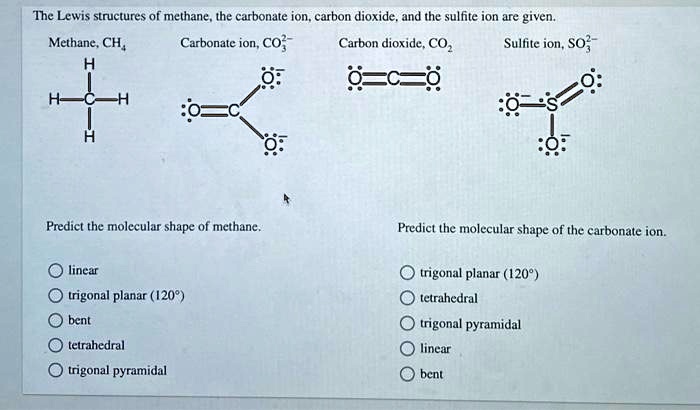
SOLVED: The Lewis structures of methane, the carbonate ion, carbon dioxide, and the sulfite ion are given. Methane (CH4), Carbonate ion (CO3^2-), Carbon dioxide (CO2), and Sulfite ion (SO3^2-) are represented. Predict
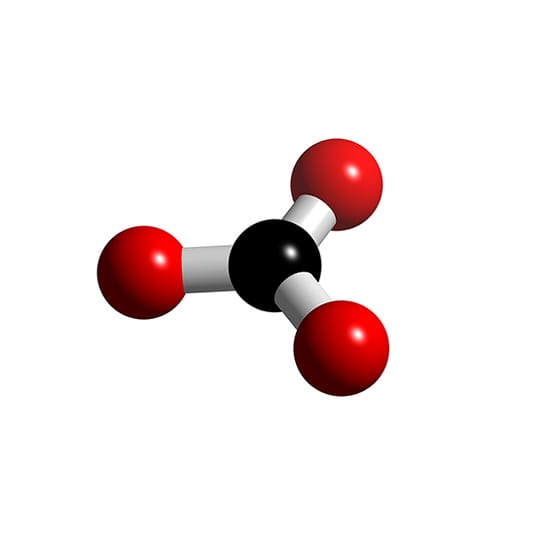
CO3]2- - Carbonate
All the C-O bonds in (CO3) 2- are equal in length. Why? - Quora

2.7 Given a carbonate ion (CO3−2) 2.7.1 Draw the

Solved Draw Lewis structure(s) showing all possible
What is the Lewis structure of CO3 2-? - Quora

Number of Lone Pairs and Bonding Pairs for CO3 2- (Carbonate ion)

Lewis Structure for CO3 2- (Carbonate ion)
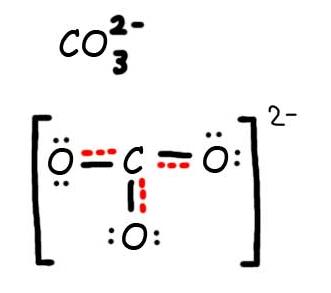
2.6: Drawing Resonance Forms - Chemistry LibreTexts

CO3 2- (Carbonate Ion) Lewis Structure

How To Calculate Bond Order Of CO32 ?

Carbonate - Wikipedia
All the C-O bonds in (CO3) 2- are equal in length. Why? - Quora