32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
$ 7.00 · 4.7 (443) · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
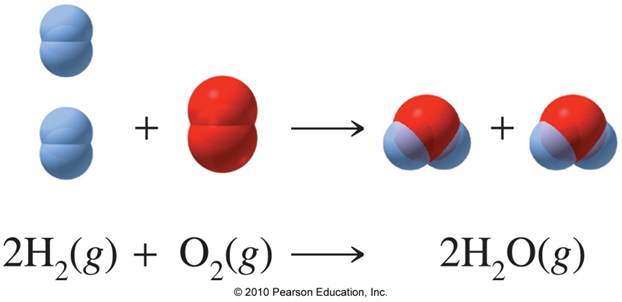
How many grams of water can be produced the combination of 8 grams of oxygen and 8 grams of hydrogen?

Spatiotemporal Decoupling of Water Electrolysis for Dual-Use Grid Energy Storage and Hydrogen Generation - ScienceDirect

Hydrogen reacts explosively with oxygen. However, a mixture of H_2 and O_2 can exist indefinitely at room temperature. Explain why H_2 and O_2 do not react under these conditions.

Malayalam] Find out the limiting reagent when 5g of H2 reacts with 24
Hydrogen Peroxide, H2O2

3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.
If 10 cm3 of each hydrogen and oxygen gases react to form water, what will the limiting reagent be? - Quora
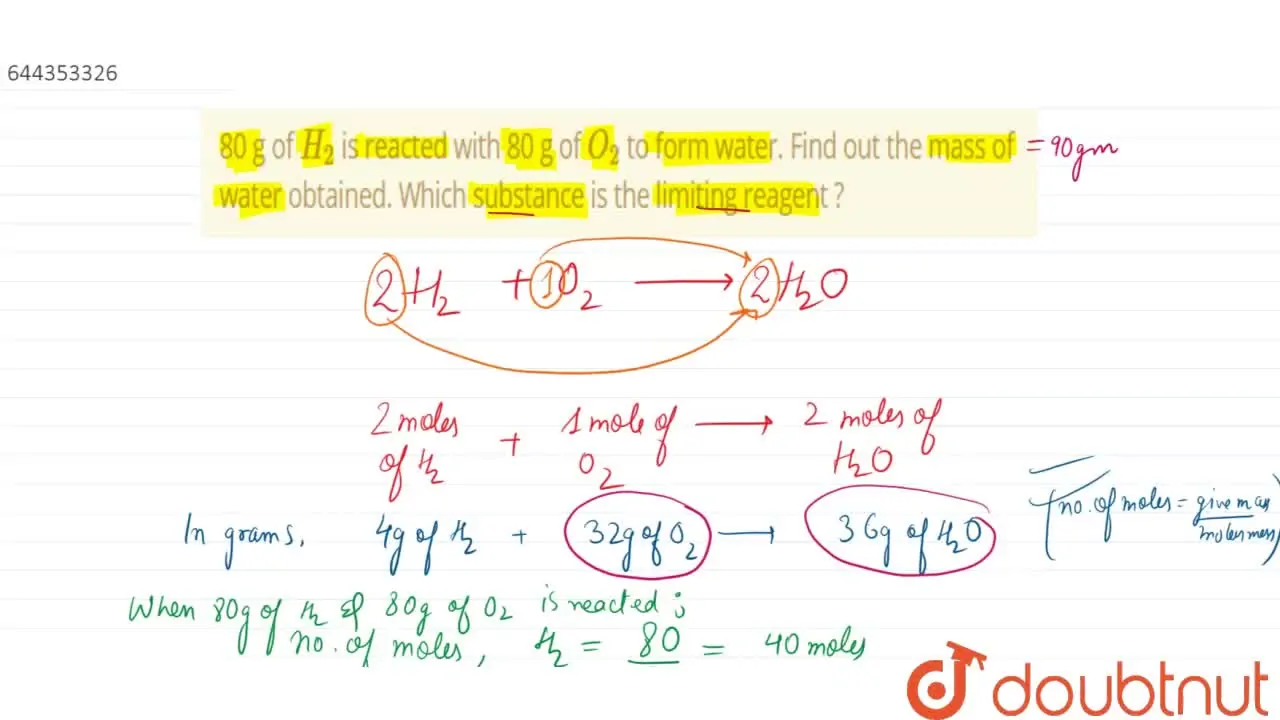
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the
22009-38-7, 7-Hydroxy-3,4-dihydronaphthalen-1(2H)-one
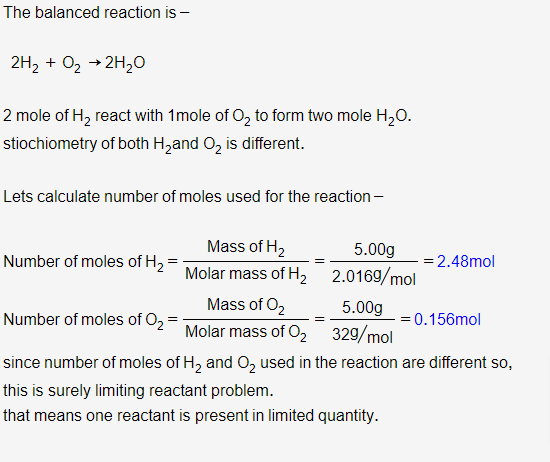
Answered: 3. Hydrogen and oxygen gas combine to…

Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water