At low pressure, the van der waal's equation is written as (P+ a/V
$ 7.99 · 5 (620) · In stock
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

Non-Ideal Gas Behavior
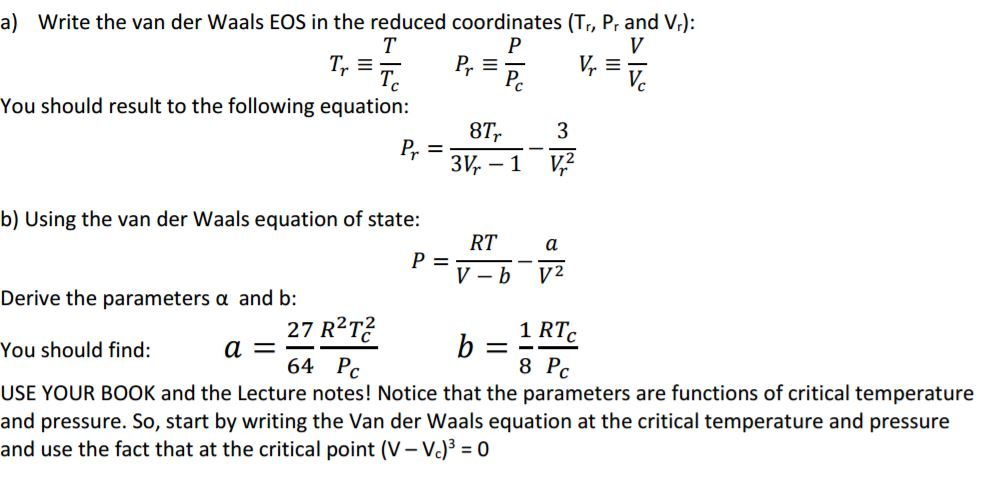
Solved Write the van der Waals EOS in the reduced
✓ Solved: van der Waals Equation Calculate the pressure of water

At low temperature if RT=2√ap , ( a is vander waal constant
At low pressure, the van der waal's equation is written as (P+ a/V

Solved The Van der Waal's equation of state is: P = nRT/V

in van der waals equation (p plus a/v square)(v-b)=RT, where p is

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0
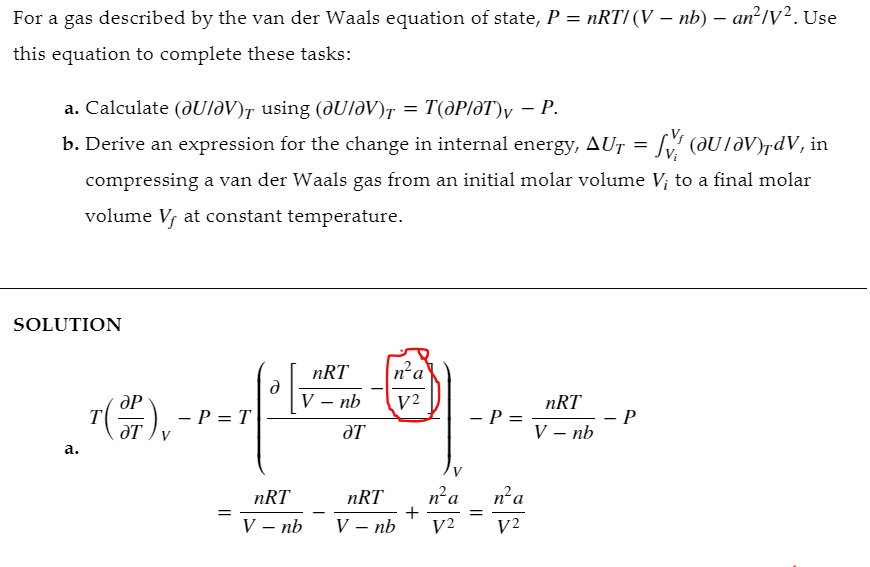
SOLVED: For a gas described by the van der Waals equation of state

P+V2a)(V−b)=RT represents the equation of a real gas and is
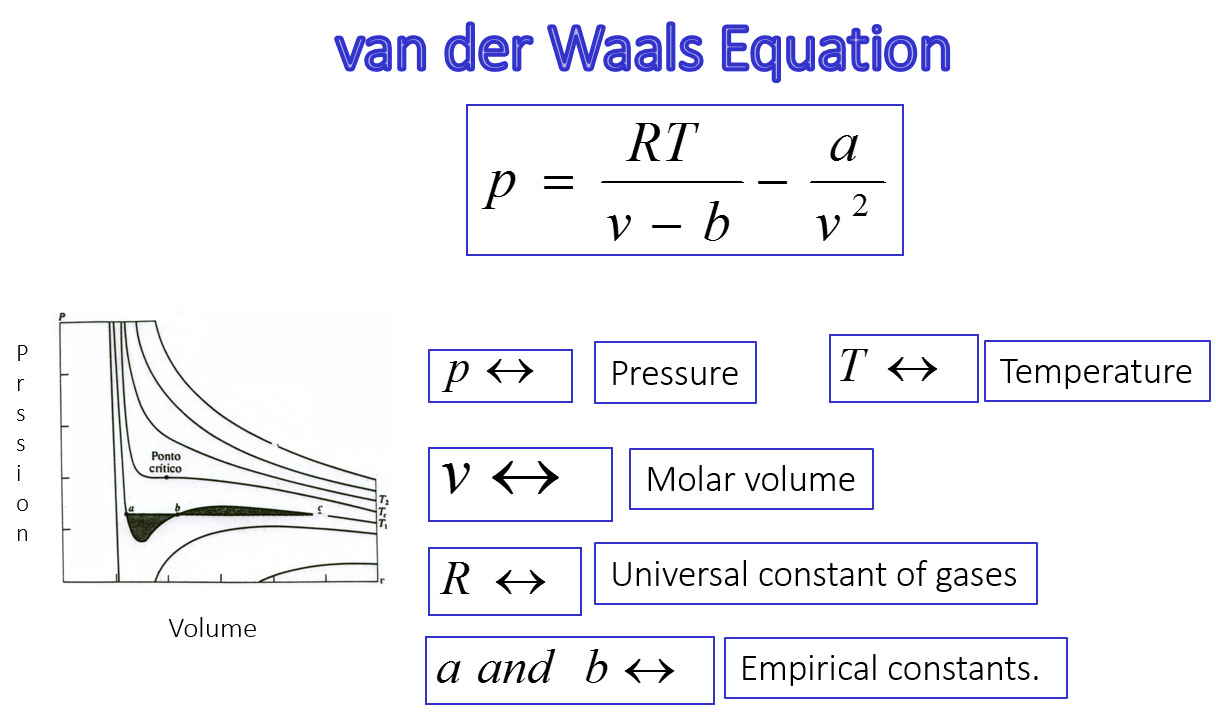
15 Extraordinary Facts About Van Der Waals Equation

In the van der wall equation : P + a/V2 (V - b) = RT

At high temperature and low pressure, the van der Waals' equation
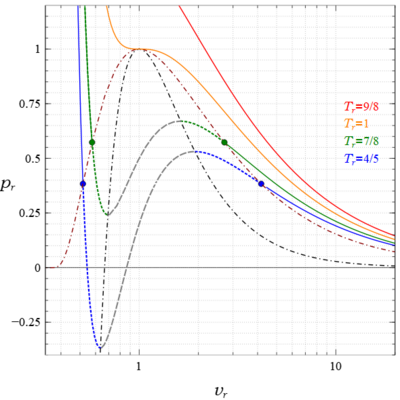
Van der Waals equation - Wikipedia