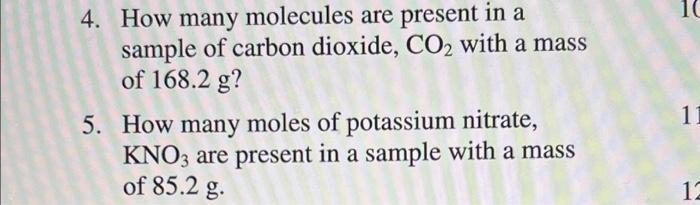What mass of carbon is present in 44g of carbon dioxide? - Quora
$ 15.50 · 4.7 (392) · In stock

Mole Concept and StoichiometryClass 10 - All About Chemistry
What is the mass in grams represented by 0.40 moles of carbon dioxide? - Quora
CaO(s) + CO2(g) → CaCO3(s) + heat What is the total mass of CO2(s) needed to produce 300. grams of CaCO3(s)? - Quora
On complete combustion with oxygen, 0.42g of a gaseous hydrocarbon (X) gave 1.32g of carbon dioxide and 0.54g of water. What is the empirical formula of the compound? - Quora
Solved 10 4. How many molecules are present in a sample of
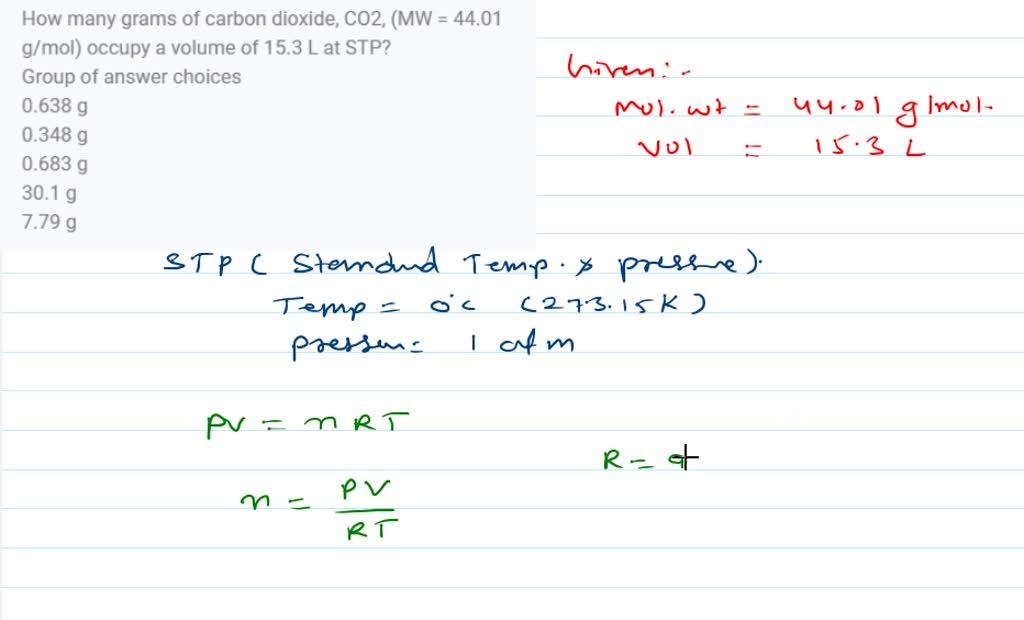
SOLVED: How many grams of carbon dioxide, CO2, (MW = 44.01 g/mol) occupy a volume of 15.3 L at STP? Group of answer choices 0.638 g 0.348 g 0.683 g 30.1 g 7.79 g
What is the volume of oxygen required to burn 36 grams of carbon completely at STP? - Quora
Why does carbon dioxide, with a molecular weight of 44.01, ascend in an atmosphere with a molecular weight of 28.97? - Quora
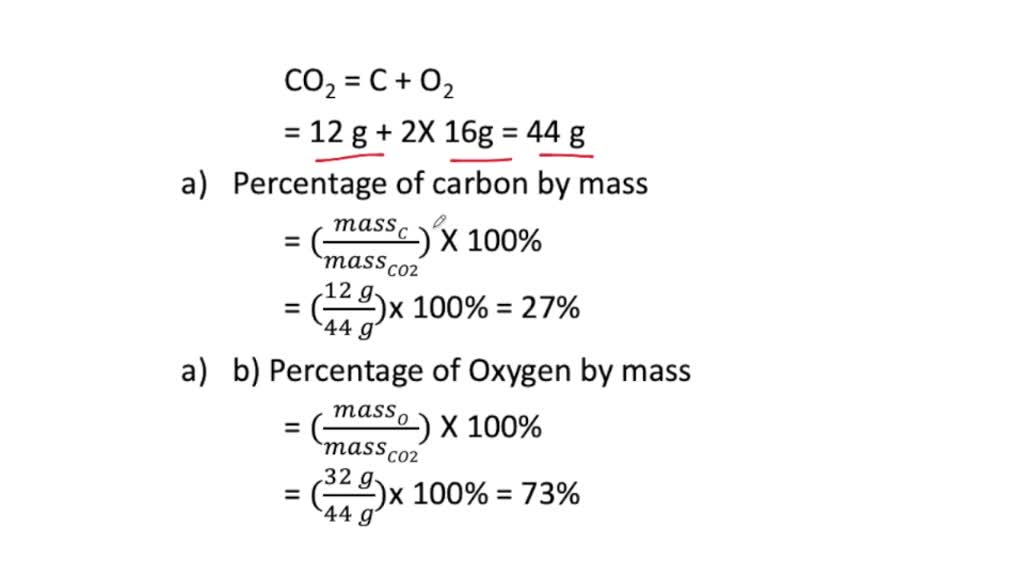
⏩SOLVED:a. What is the percent by mass of carbon in 44 g of carbon…

Why does carbon dioxide, with a molecular weight of 44.01, ascend in an atmosphere with a molecular weight of 28.97? - Quora

How to Find the Mass of One Molecule of Carbon dioxide (CO2)