The role of the compressibility factor Z in describing the
$ 32.00 · 4.9 (284) · In stock
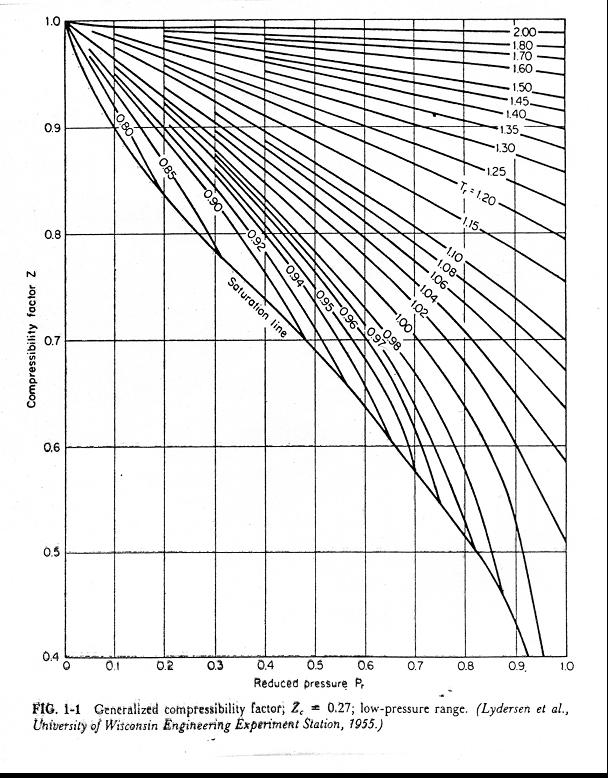
In this post I will give a recapitulation of the role the compressibilty factor Z plays in the volumetric behavior of gases. The purpose of this post is also to give some background to the first post of June , 2013 describing a compact, explicit equation for the vapor compressibility factor Z in the sub-critical…
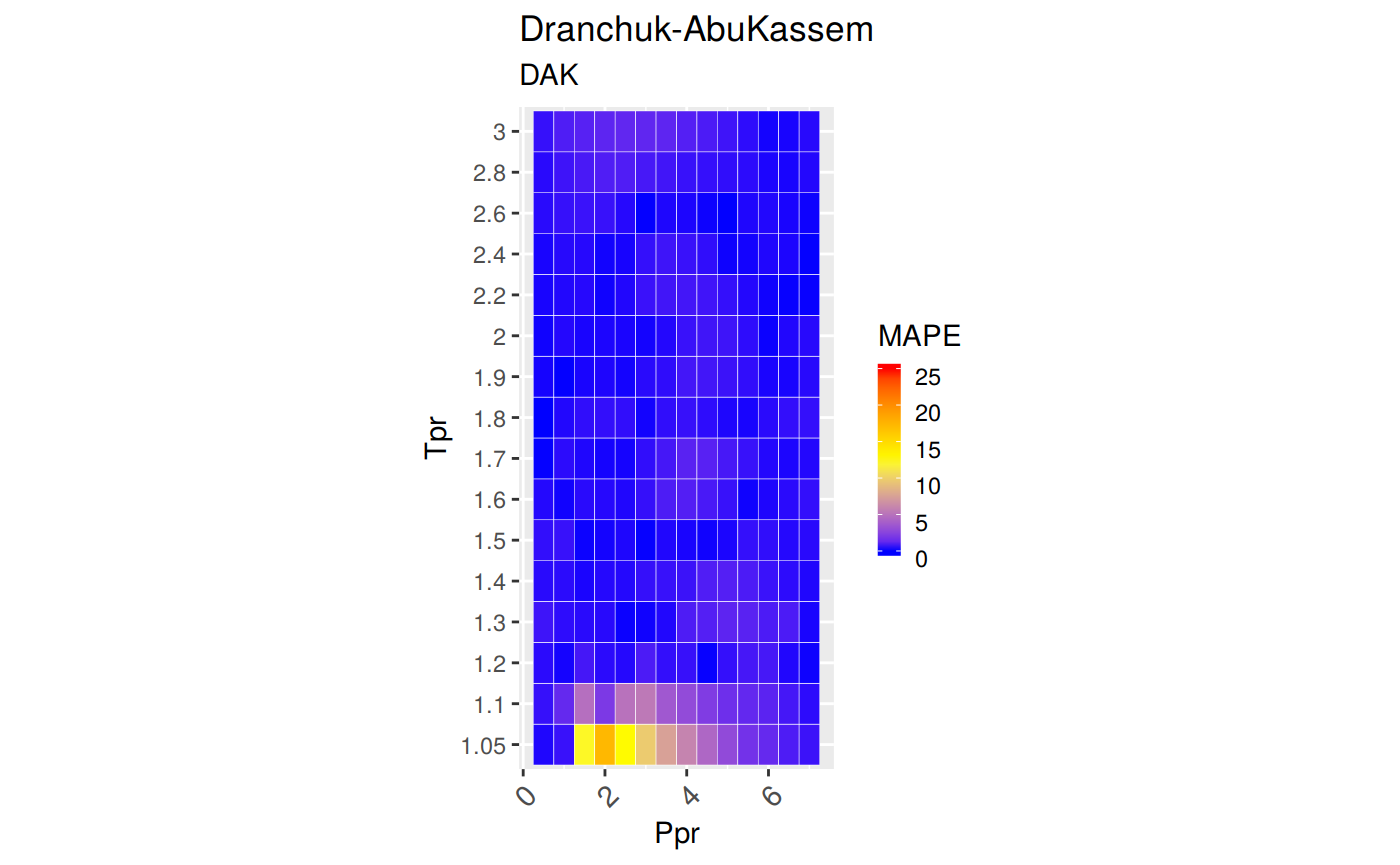
Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

SOLVED: What is the physical significance of the compressibility factor Z ?

Determine Compressibility of Gases

Variation of the compressibility factor, Z (solid curves), of CO 2 as a
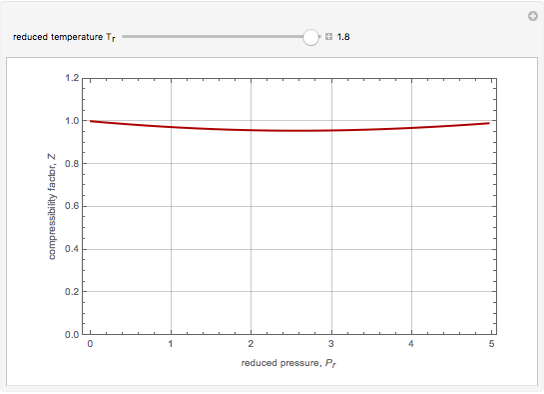
Compressibility Factors Using the Soave-Redlich-Kwong Equation of State - Wolfram Demonstrations Project

Improved description of the liquid phase properties of Methane: density, enthalpy, plus saturated vapor compressibility factor

Compressibility Factor Z // Thermodynamics - Class 85

Objectives_template

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

ars.els-cdn.com/content/image/3-s2.0-B978012809597

Class Notes on Compressibility of a Real Gas, CH 417, Study notes Physical Chemistry
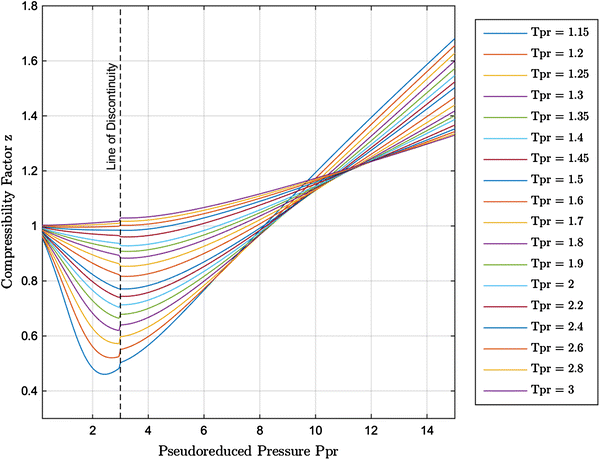
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms