Elacestrant Impresses in EMERALD Trial of Breast Cancer
$ 6.99 · 4.8 (473) · In stock


Elacestrant in metastatic breast cancer: Is the “standard of care” meeting standard requirements? - ScienceDirect

EMERALD Phase 3 Trial of Elacestrant Versus Standard of Care Endocrine Therapy in Patients With ER+/HER2- Metastatic Breast Cancer: Updated Results by Duration of Prior CDK4/6i in Metastatic Setting

Addressing Skin-Related Toxicities Extends Positive Clinical Outcomes
Lorenzo Gerratana on X: First results of the EMERALD trial: single agent # elacestrant is a promising new endocrine option, #biomarkers are needed for a real #personalized treatment there is a rapidly progressive

EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer
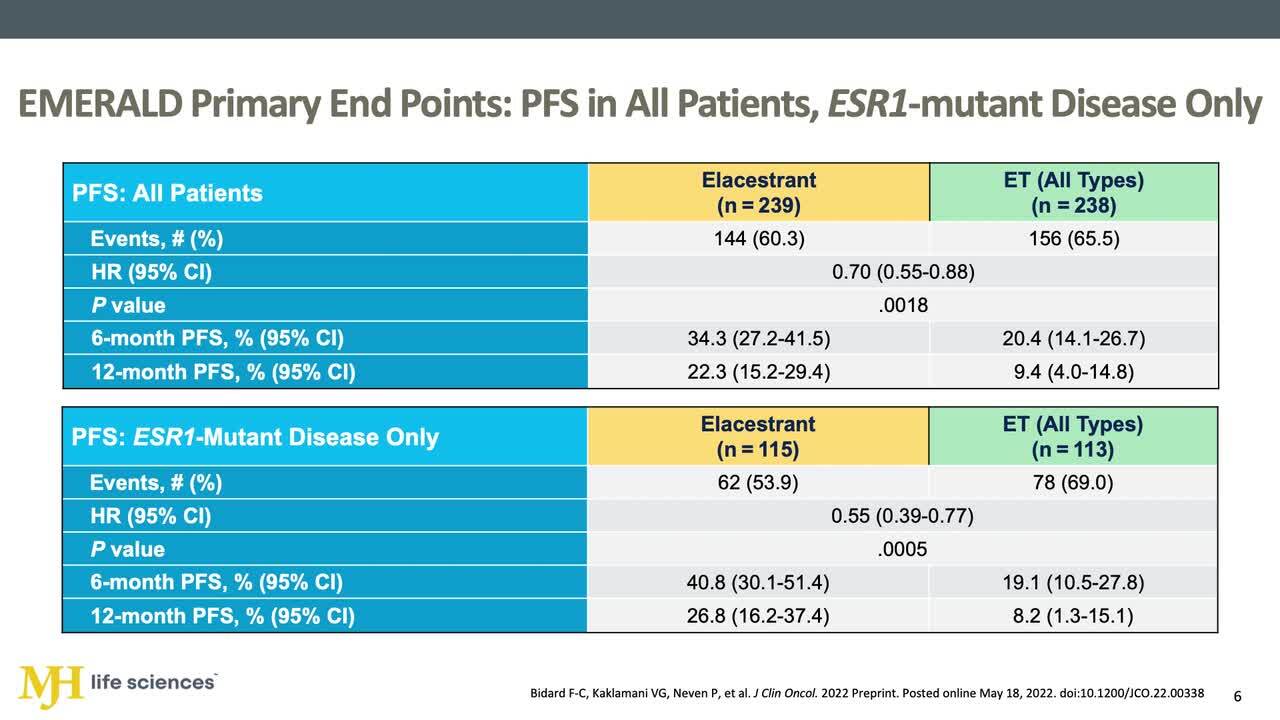
Elacestrant (Oral Selective Estrogen Receptor Degrader) versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results from the Randomized Phase III EMERALD Trial
Pipeline Report 2022: Patient experience takes center stage

Breast Cancer, Oncology News & Insights, Targeted Oncology

Dr. O'Shaughnessy on Key Results From the EMERALD Trial in HR+/HER2- Breast Cancer
![]()
How are people with liver mets doing? - Page 654 — Breastcancer.org
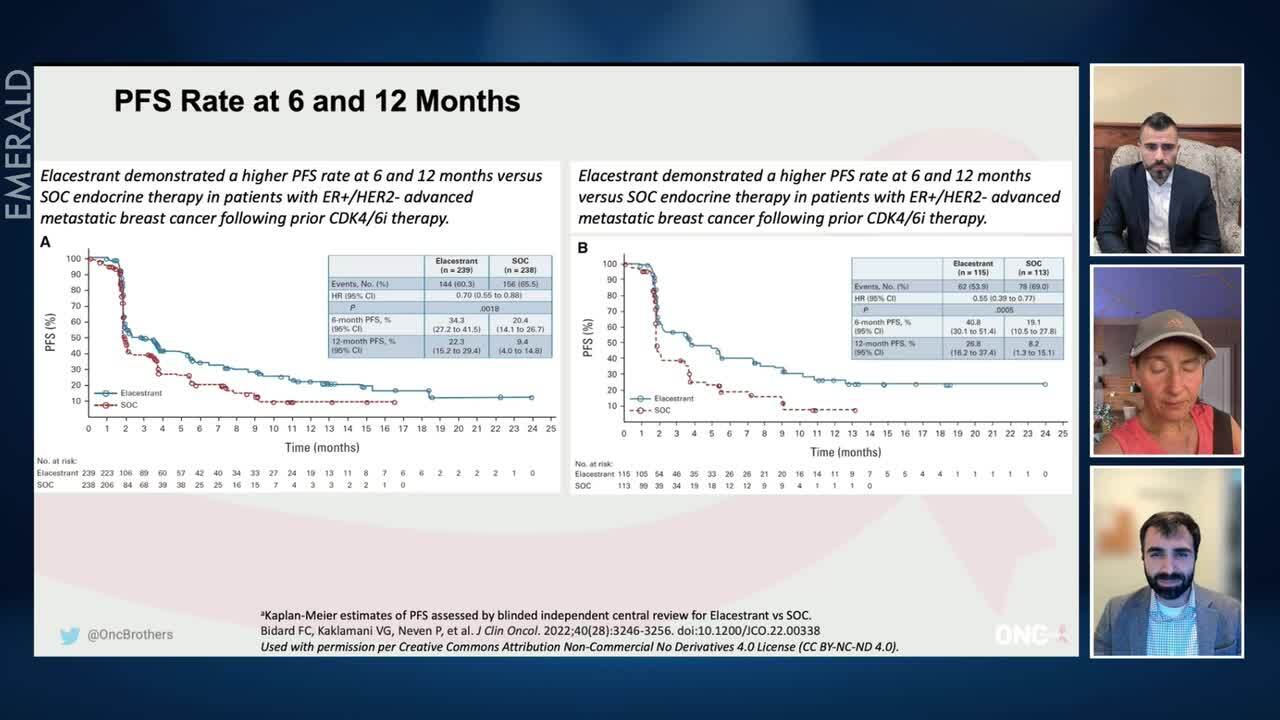
EMERALD Trial of Elacestrant in ER+, HER2- Advanced Breast Cancer: Efficacy and Safety Data

Breast landmark trials dr.kiran

EMERALD Trial ORSERDU™ (elacestrant) Efficacy

Promising Phase III Results in HR+/HER2- Breast Cancer
