Compressibility factor (Z) for a van der Waals real gas at critical point is
$ 20.00 · 4.8 (67) · In stock

Share your videos with friends, family and the world

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced
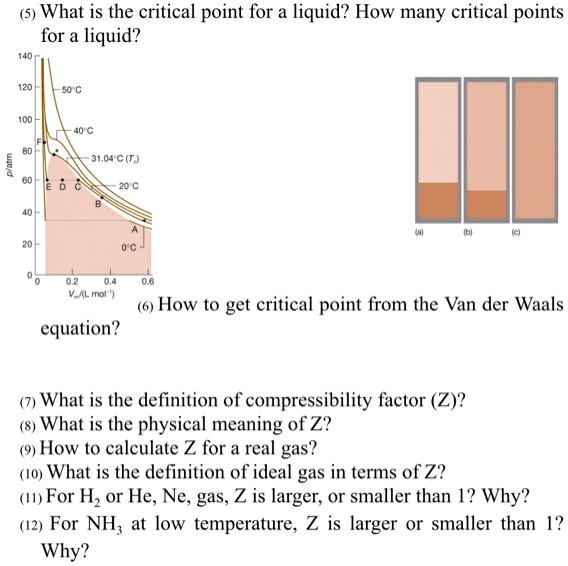
SOLVED: What is the critical point for a liquid? How many critical points for a liquid? 5 31.04 VcALmol How to get the critical point from the Van der Waals equation? (
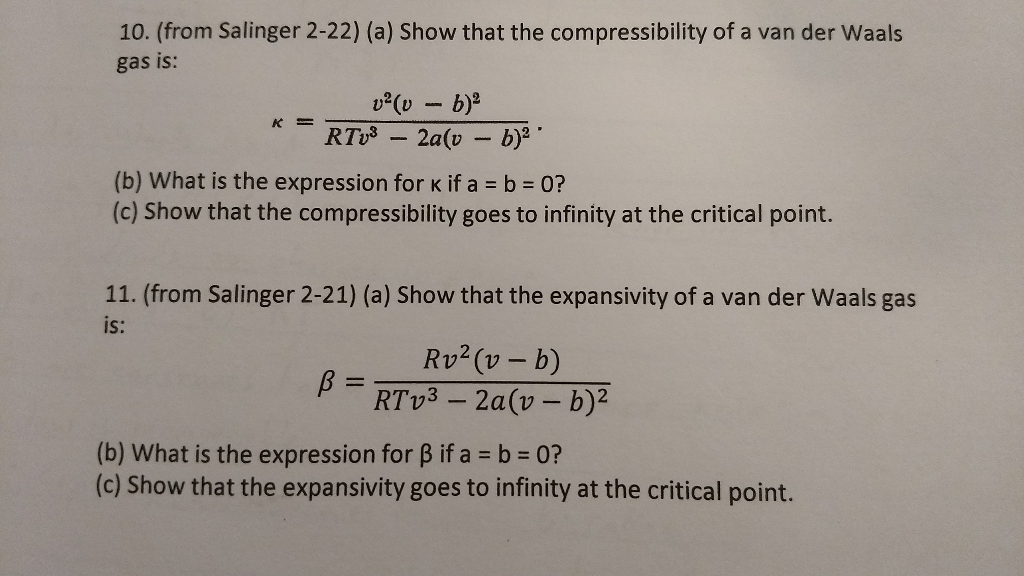
Solved (a) Show that the compressibility of a van der Waals

The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6

PDF) A Modified Form of the van der Waals Equation of State

The compressibility factor Z of one mole of Vander Waals gas with negligible 'a' value is a) bp/RT b) [1-(bp/RT) c)[1 (bp/RT) d) (1/bp)? - EduRev NEET Question
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
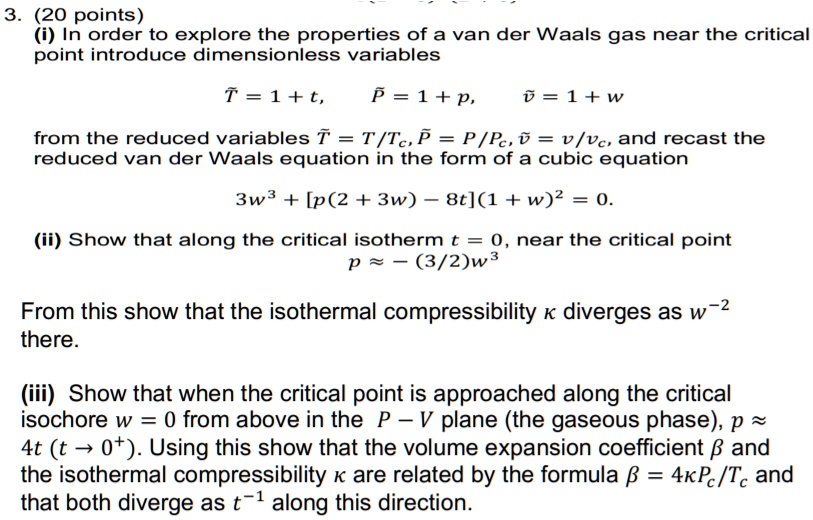
SOLVED: (i) In order to explore the properties of a van der Waals gas near the critical point, introduce dimensionless variables: T = 1 + t, P = 1 + p, V =

The Compression Factor, Z, and Real Gases - What you NEED to Know!

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility factor - Wikipedia

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
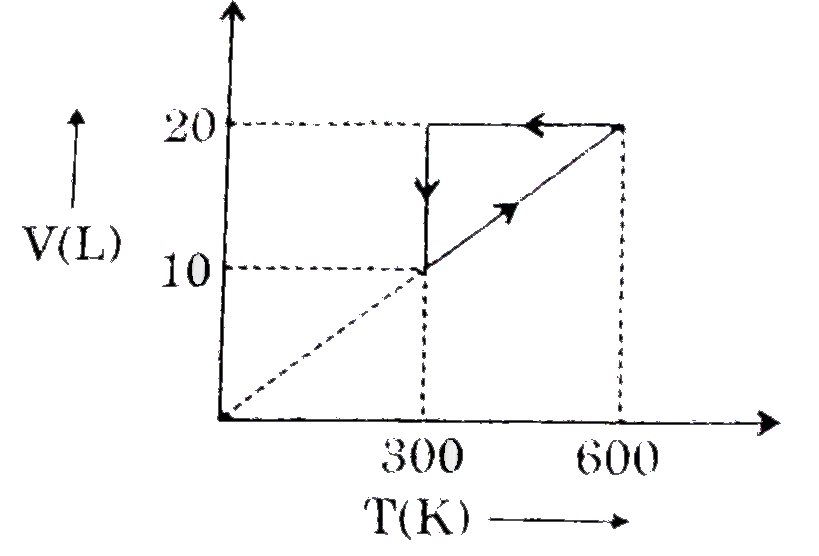
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

Maxwell's speed distribution curve