200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
$ 4.50 · 4.9 (438) · In stock
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

Calculate the weight of lime (CaO) obtained by heating 200 kg of 95% pure lime stone `(CaCO_(3)).`
When a limestone of mass 150g was heated until it decomposed to CaO, only 63g of CaO were obtained. What is the percentage purity of the limestone? - Quora
Chem o Level Notes, PDF, Redox
When a limestone of mass 150g was heated until it decomposed to CaO, only 63g of CaO were obtained. What is the percentage purity of the limestone? - Quora

58. 50 g of a sample of limestone (CaCO3) on complete 58 decomposition gives 20 g of CO2. The percentage purity of CaCO3 in limestone is (Atomic mass of Ca = 40 u) (1) 75% (2) 85% (3) 95.2% (4) 90.9% 0
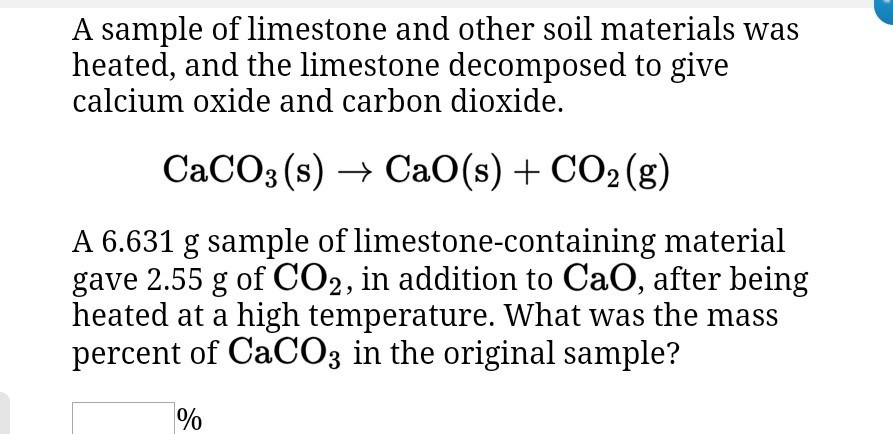
Solved A sample of limestone and other soil materials was
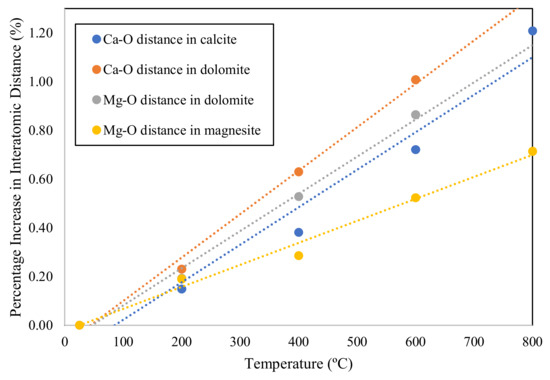
Applied Sciences, Free Full-Text

⏩SOLVED:A sample of limestone (containing calcium carbonate, CaCO3 )…

ESTERFIP, A TRANSESTERIFICATION PROCESS TO PRODUCE

Ranchers' big blast shatters copper orebody for - University of Utah
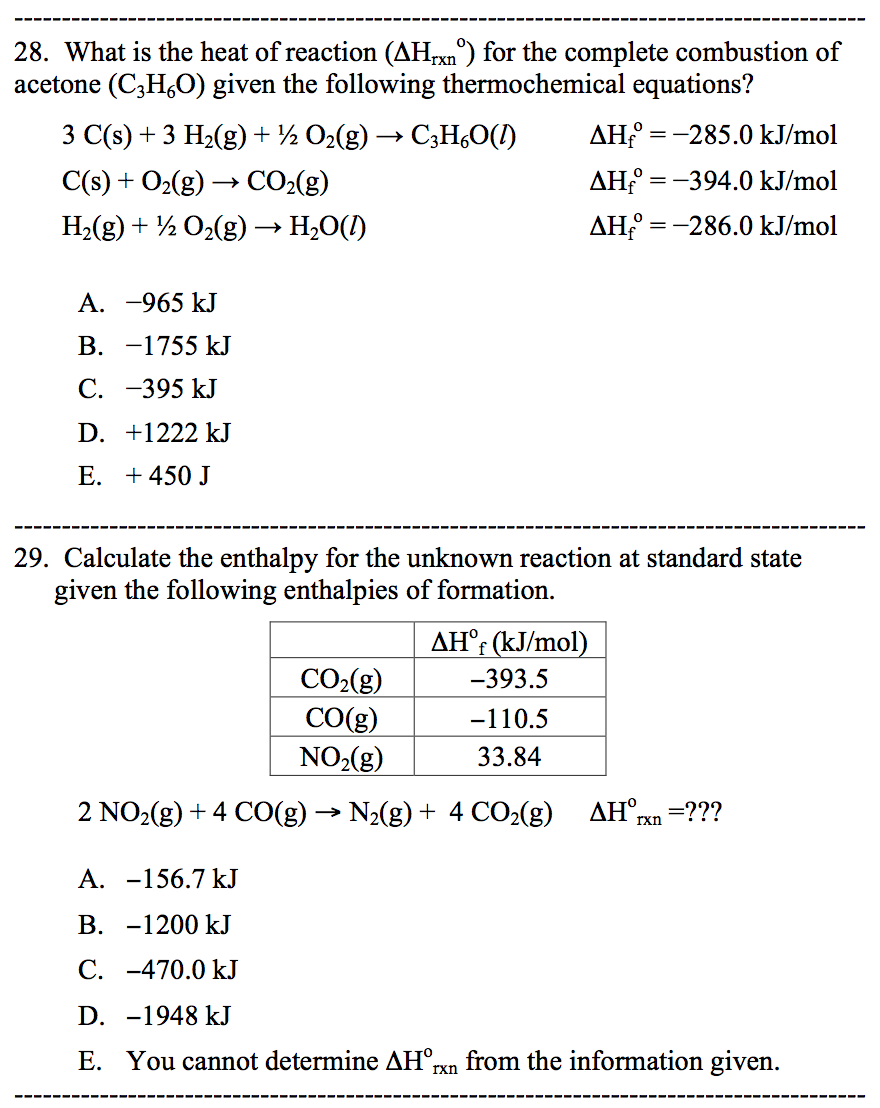
Solved Please help me solve the following questions below

0958 ch11.pdf - Index of - Free

Applied Sciences, Free Full-Text
